Changes to AP Chemistry is a big deal, even if you aren’t a teacher or student. Many future chemists get their first taste of chemistry in an AP class or an introductory class influenced by AP Chemistry. Many high school teachers attend AP Chemistry workshops even if they don’t teach AP. And many AP Chemistry students will find themselves in college chemistry courses (Organic for those who pass, General for those who do not). The AP students I get in General Chemistry always set the curve in my class.
So when the College Board (which runs AP) re-writes the curriculum, it’s a big deal for the entire chemistry community.
The new curriculum is a response to a 2002 report from the National Academies called Learning and Understanding: Improving Advanced Study of Mathematics and Science in U.S. High School. You can read it for yourself (it’s free if you sign up for an account, but it is 300 pages..), but the gist is: AP science (1) covers too much content while skimming the practice of science and (2) spends too much time on rote methods for problem solving, what I call “calculator monkey” work. College Board took the critique seriously and began writing a curriculum that was more oriented toward science practices and less toward calculator monkey sorts of chemistry problems.
The new curriculum (you can read the whole 109 pages here) is rooted in the chemical education literature, namely, it stresses inquiry-based approaches to teaching and stresses that students should be able to shift back and forth between different representations of a problem (particulate, mathematical and macroscopic/lab observations). I will explore this aspect more deeply in a future post.
So what’s different about this new curriculum? The first difference is in style. The course is built around 6 “Big Ideas” and 7 “Science Practices”. For example:
Big Idea 2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molecules and the forces between them.
And
Science Practice 1: The student can use representations and models to communicate scientific phenomena and solve scientific problems.
Each of the Big Ideas and Science practices are subdivided into more specific statements, but I’ll gloss over the details for now. The idea is that Big Ideas and Science Practices riff off each other to produce a set of learning objectives – things that a student in AP Chemistry should be able to do. Take an example:
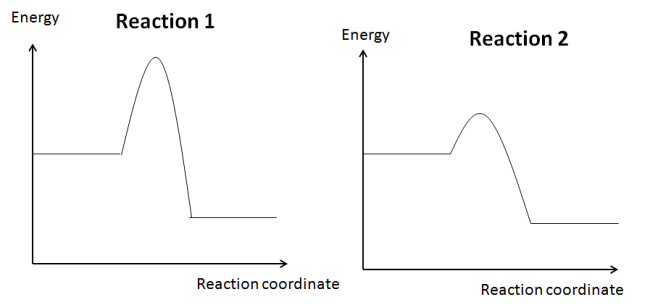
Learning Objective 4.6 The student is able to use representations of the energy profile for an elementary reaction (from the reactants, through the transition state, to the products) to make qualitative predictions regarding the relative temperature dependence of the reaction rate. [See Science Practice 1.4, 6.4]
Welcome to curriculum-speak. In average chemist terms, that means a student should be able to use a graph like the following to explain why a reaction is fast or slow.
The fact that Reaction 1 has a greater difference in energy between the reactants and the transition state (i.e., activation energy) means that it will be slower than Reaction 2.
Incidentally, Science Practices 1.4 and 6.4 are:
Science Practice 1.4 The student can use representations and models to analyze situations or solve problems qualitatively and quantitatively.
Science Practice 6.4 The student can make claims and predictions about natural phenomena based on scientific theories and models.
So the student should be able to go back and forth between using the graph (which is the model) and a macroscopic observations (the speed of the reaction). Thus, on the AP test a student might be asked to first analyze a set of kinetics data, then draw an energy diagram that matches the data. Or the student could be given an energy diagram and asked to correctly associate the graph with concentration versus time data.
Probably the biggest difference is the emphasis on concepts rather than mathematics. The rationale, coming the National Academies report, is that students often memorize mathematical routines without understanding the underlying concept. My favorite example of this is that questions regarding the Nernst equation will no longer be included on the AP exam. Instead, students will be asked to use LeChatlier’s principle to reason about whether the potential of an electrochemical cell will increase or decrease based on the concentration of reactants and products (Learning Objective 3.12).
Personally I think this is a great change. My undergraduate mentor Vicki Colvin always made the point that we would forget the math but remember the concepts. And she was write. I can’t solve the Schrödinger equation from memory anymore, but I really understand wave-particle duality (well, as much as anyone understands it). However, many people are pretty upset about this. I’ve been in more than one presentation where people started yelling. The feeling (which is reasonable) is that we are dumbing down AP Chemistry. I think that is not fair. Take for example Learning Objective 6.17.
LO 6.17 The student can, given an arbitrary mixture of weak and strong acids and bases (including polyprotic systems), determine which species will react strongly with one another (i.e., with K >1) and what species will be present in large concentrations at equilibrium.
No where do the Learning objectives say that students should be able to calculate the pH at an arbitrary point on a titration curve. But frankly, the above is just as hard (notice the word “arbitrary”!), requires more understanding than memorization, and is more useful to a student going on to Organic Chemistry anyway!
The new curriculum is going to take a while to digest, and a few years of difficult adjustment, as in painful-to-watch-butterfly-struggling-out-of-a-chrysalis kind of difficult adjustment. It is my goal to write a series of posts on this blog that help explain the curriculum in more depth, both for AP teachers and chemists in general. It is my hope that these posts help alleviate just a bit of that adjustment.
Thoughts and Comments? Either comment below or find me on Twitter @SGPrilliman.
Disclosure: I am an AP Chemistry consultant and a reviewer of AP Audit syllabi.
Is there a new AP Lab Manual that accompanies this Curriculum Document? I would love some more lab ideas to add to my repertoire. Thank you
Yes, there are two sources of labs that I know of. The first is the College Board issued its own manual. There is information here about how to get one:
http://apcentral.collegeboard.com/apc/members/courses/teachers_corner/221821.html
Also, some members of the POGIL project (including myself) are working with PASCO on a manual. It’s not complete yet, but you can get samples at http://www.pasco.com/elements.
I live in China and my daughter is homeschooled here. She’s a junior, taking AP Chem this year and will take the AP exam next year. We’re using a video AP course from Thinkwell. I – unfortunately – just today for the first time heard about the changes in the AP curriculum—by chance, when I saw your post while searching online for something else. Needless to say, it was disheartening news since the course she’s taking no doubt reflects the old curriculum not the new one (and the “latest” Barrons AP book we ordered from the US is also outdated—too bad that wasn’t mentioned on the Amazon page). My question is, do you have any recommendations of resources (books or course) that my daughter could study, in addition to the AP Chem course she’s currently taking, to make sure she’s familiar with any NEW topics covered in the new curriculum? Or, if no new topics are introduce with the curriculum changes, are there any books that explain things in a way that’s similar to how the new AP curriculum is structured (with the same focus on “concepts”), which she could at least read through to make sure she’s conceptually seeing things the way the new AP curriculum does? Thank you for your time.
Hi Sean,
The new curriculum is very different from the old one. Studying materials for the old exam will not sufficiently prepare any student for the 2014 exam.
I wish I had good review books to recommend, but the ones I saw last summer were not as good as I would have liked and I couldn’t recommend any of them. Bascially everyone (teachers, textbook and review book publishers, etc.) are trying to keep up with the changes but somehow everyone seems to be a little behind.
The only textbook I can recommend is Spencer and Bodner’s book Chemistry: Structure and Dynamics. The authors are all part of the chemical education communinity that helped redesign the curriculum.
Best of luck!
SP
Hello!
Do you think the students will do better on this revised version of the exam? Do you think the curve will go up, or down?
I just took the exam, and I thought the FRQ part was quite different from the previous ones…as you said, very conceptual.
Also, I realized that there were two different exams, so not everybody took the same one; it seems that one was harder than the other.
Do you think that is possible?
Sorry for asking too many questions at once; I am just so confused by all these changes and do not have any idea what score I would get. I am sincerely hoping the curve would be good, but I am not sure.
Thank you,
Eleanor
Hi Eleanor,
Congratulations on finishing the exam! I’m not sure what will happen with score distribution. When AP Biology went through their exam revision a coupld of years ago the number of students getting 3, 4 or 5 grades decreased a little bit. I wouldn’t be surprised if this happened for AP Chemistry as well. The AP exam standards are set by comparing the AP exam raw scores (percent right) versus the scores of college students in General Chemistry, some of whom take a similar AP exam as their final. The idea is that if a students gets an A in university General Chemistry, a student making a comparable score on the AP Chemistry exam should get a 5. This keeps everything fair because it means that if a student gets a 2 on the AP exam, that student wasn’t prepared to pass General Chemistry and needs to retake the course anyway.
Either way, it’s out of your hands now, so try not to worry about it. Best of luck to you, and I hope you learned a ton of chemistry this year and hope you get the score you’re looking for!
Do you know of any online AP courses that have changed sufficiently to meet the requirements of the new AP in Chemistry or can you yet recommend any video type series or self-study program?
No, I’m afraid I do not. I have little expertise in the area of online instruction for high schools. There are supposedly some colleges that offer online General Chrmistry courses and those may be more appropriate for your needs, assuming the courses are taken from an accredited institution that has a good track record of acceptance of credit with your anticipated university.